 aide
aide
REVISITSHAPE
RNA is an essential macromolecule with diverse functions that mainly depend on its ability to fold into higher order structures. RNA serves as the genome for many pathogenic viruses. In addition to its role as messenger between DNA and the ribosome in bacteria and eukaryotes, RNA is involved in numerous fundamental cellular processes such as gene regulation and host defense. Note that many RNA functions are still unknown. This point can be illustrated by the recent discovery of thousands of long non-coding RNAs (lncRNAs, from 200 to 10,000 nucleotides in general) in mammalian species (1). A study estimates
approximately 1 5,000 lncRNAs in humans (2). Most lncRNAs play key roles in development, epigenetics, cancer, brain function and hereditary diseases (3) but fundamental questions regarding the functions of lncRNAs remain unanswered.
RNAs show a hierarchical organization in which the primary sequence determines the secondary structure (the pattern of base pairing) that governs the tertiary folding. RNA functions may imply sequence, secondary and tertiary structures, or a combination of these features. The crystallographic and NMR approaches have led to determine the threedimensional structures of several functional RNAs but the high-throughput use of these methods is limited, especially for long RNAs. Interestingly, secondary structures, usually
more stable than tertiary interactions, are usually considered as the key point for RNA functions.
An extensive understanding of the functions of RNAs in general and lncRNAs in particular requires an important effort to improve our knowledge of their structures, folding and dynamics, especially concerning their secondary structure. Our project will contribute to improve RNA secondary structure determination.
RNA SHAPE Chemistry with Aromatic Acylating Reagents
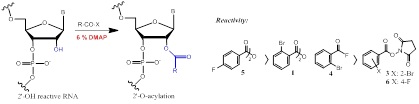
As chemical methods for RNA secondary structure determination, SHAPE chemistry (selective 2'-hydroxyl acylation analyzed by primer extension) has been developed to specifically target flexible nucleotides (often unpaired nucleotides) independently to their purine or pyrimidine nature. This study is the first to show that nucleophilic catalysts like DMAP greatly improved the selective 2'-hydroxyl acylation by symmetric anhydrides, acyl fluorides and succinimidyl ester.
approximately 1 5,000 lncRNAs in humans (2). Most lncRNAs play key roles in development, epigenetics, cancer, brain function and hereditary diseases (3) but fundamental questions regarding the functions of lncRNAs remain unanswered.
RNAs show a hierarchical organization in which the primary sequence determines the secondary structure (the pattern of base pairing) that governs the tertiary folding. RNA functions may imply sequence, secondary and tertiary structures, or a combination of these features. The crystallographic and NMR approaches have led to determine the threedimensional structures of several functional RNAs but the high-throughput use of these methods is limited, especially for long RNAs. Interestingly, secondary structures, usually
more stable than tertiary interactions, are usually considered as the key point for RNA functions.
An extensive understanding of the functions of RNAs in general and lncRNAs in particular requires an important effort to improve our knowledge of their structures, folding and dynamics, especially concerning their secondary structure. Our project will contribute to improve RNA secondary structure determination.
RNA SHAPE Chemistry with Aromatic Acylating Reagents
As chemical methods for RNA secondary structure determination, SHAPE chemistry (selective 2'-hydroxyl acylation analyzed by primer extension) has been developed to specifically target flexible nucleotides (often unpaired nucleotides) independently to their purine or pyrimidine nature. This study is the first to show that nucleophilic catalysts like DMAP greatly improved the selective 2'-hydroxyl acylation by symmetric anhydrides, acyl fluorides and succinimidyl ester.
